Intro
ClearSight LLC is proud to announce the ClearSight Intraocular Lens — a revolutionary device designed to give ophthalmologists a comprehensive solution to reduce posterior capsule opacification. The ClearSight IOL features Sharklet, a micropattern designed to redirect cellular migration away from the field of vision.
The ClearSight IOL has distinct features each designed for a specific purpose:
-
The lens platform easily integrates with any premium optic.
-
The material is specifically formulated to be free of glistenings.
-
The outer portion of the lens has a 360⁰ square-edge micropatterned membrane preventing LEC migration responsible for PCO formation.
Cataracts
According to the World Health Organization, cataract is the leading cause of blindness, with over 20 million people affected in 2010. While age is the leading cause, younger people can develop cataract through eye disease or injury.
Current treatment for cataracts is simple: the clouded lens is removed, and a new, artificial intraocular lens is inserted. After the surgery, cells migrate onto the new lens, making it cloudy. A followup procedure uses an Nd:YAG laser to remove the migrated cells. Unfortunately, these laser procedures are expensive and available to less than 1% of the world’s population. In 2012 alone, Medicare reimbursed more than $350 million for laser capsulotomy surgeries.

Technology


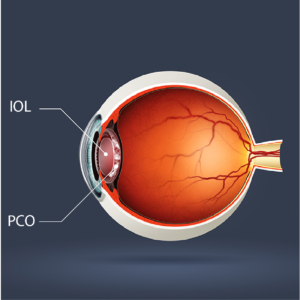
Versatile IOL Platform
With the growing prevalence of premium optics (extended depth of focus, multifocal), patient expectations regarding visual outcomes are increasing. The ClearSight PCO-preventing platform can be integrated with the full range of premium optics—allowing for maximum patient satisfaction for those that have paid a premium out-of-pocket for their IOL implant.
Anti-Glistening Material
The ClearSight IOL is made in a patented hydrophobic acrylic material that is formulated to eliminate glistenings. Currently, the two market-leading IOLs in the U.S. are plagued by the occurrence of glistenings in the material, which can sometimes affect visual quality. Our material eliminates this issue, providing a better platform for delivering a premium extended depth-of-field optic.
Designed to Prevent Lens Opacification
The ClearSight IOL is designed to prevent PCO. A novel square-edge membrane fully surrounds and protects the optic against LEC migration. The membrane is printed with Sharklet, a micropattern designed to inhibit the migration of cells onto the new lens. In vitro studies revealed an 80% reduction in LEC migration, while in vivo studies demonstrated 100% reduction in visually significant PCO in rabbit eyes. These results signify a breakthrough in PCO prevention.
Research
-
This piece, published in Translational Vision Science & Technology, explains the effect that the Sharklet micropattern has on cells that cause PCO
-
For more information about Sharklet, visit www.sharklet.com
-
In this in vivo study, experts at the University of Utah show that PCO was reduced in rabbits with 100% reduction of clinically significant PCO.
-
For more information, visit the Journal of Cataract and Refractive Surgery.
Team






Contact Us
Do you have questions or comments about ClearSight? We’d love to hear from you. Use the form below or contact us using the information to the right.
Contact Information
Address
12635 E. Montview Boulevard
Suite 136
Aurora, CO 80045
Phone
720.859.4125
Web
